There are many factors that affect the permeability of rubber. Polymer and fillers directly affect the rate of transfer through a rubber barrier, which is very important for critical gas or fluid application. The most common example of this type of transfer is air permeation through an automotive tire. Having the right barrier inside the tire is critical for overall tire performance on both hot or cold days.
For rubber, permeation is the rate at which small molecules of a gas or liquid transfer through a rubber compound. These rates are typically very low but are important when designing a critical seal.
Critical sealing applications include seals for medical instruments that go through autoclaving and cannot be affected by moisture for internal components, automotive fuel seals that must meet the California Resource Board (CARB) for low-emission vehicles, or vacuum seals that must not lose vacuum with air permeation.
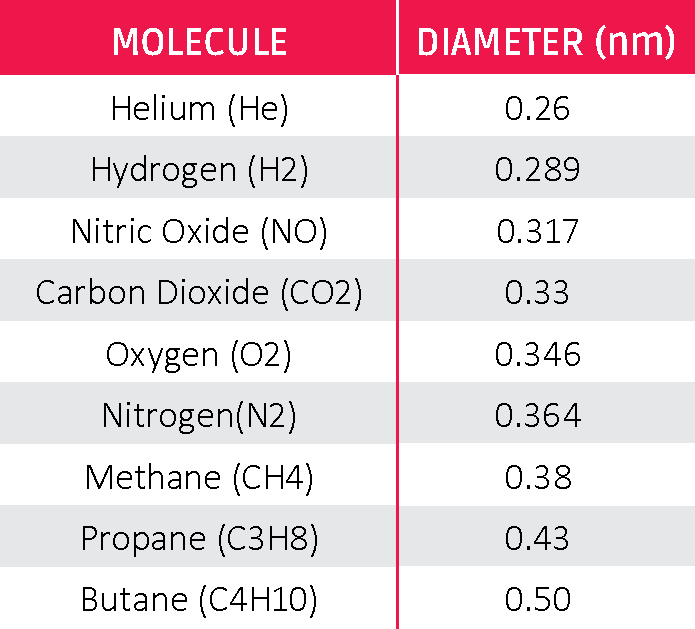
Size of the permeant molecule affects permeability rates. Kinetic diameter is the smallest effective diameter of a given molecule, assuming it’s spherical. This does not always correlate with molecular weight. Higher molecular weight can have a smaller diameter.
The polarity of the molecule and steric hindrance of the structure are factors in the size of the molecule. Table 1 lists the kinetic diameter of common gases.
Table 1: Common Kinetic Diameters
EPDM compounds have a very low absorption to water. These compounds work well as a barrier to water vapors. However, EPDM will absorb oil and gas, therefore it is not an efficient overall barrier.
Temperature is another factor that complicates permeability. Typically, the higher the temperature, the higher the rate of diffusion. Thus, it is recommended to make sure testing is done at all operating ranges.
Other factors affecting permeability are the concentration of the permeant at the barrier and pressures at the opposite side of the barrier.
Units of Measure
The units of gas and liquid permeability are shown below:
- P=(amount of gas x thickness of membrane)/(area of membrane x time x pressure)
- P=(amount of liquid x thickness of membrane) / (area of the membrane x time)
Test Methods
- ASTM D814 – Standard Test Method for Rubber Property – Vapor Transmission of Volatile Liquids
- ASTM D1434 – Standard Test Method for Determining Gas Permeability Characteristics of Plastic Film and Sheeting
- ISO 2782-1 – Rubber, vulcanized or thermoplastic — Determination of permeability to gases — Part 1: Differential-pressure methods
- ISO 2782-2 – Rubber, vulcanized or thermoplastic — Determination of permeability to gases — Part 2: Equal-pressure method
Figure 1: Permeability Cup
Rubber Formulation Considerations
Filler particle size. Carbon black and clay fillers have various mean particle size. Small particles typically give higher physical properties, but can adversely affect processing. Compound viscosity increases with higher loading of small particle fillers, which can cause mold flow issues. Making the filler more dense with high loading of smaller filler particles helps prevent molecules from traveling through.
Filler absorption. Some fillers will absorb the permeant. Clay fillers can absorb water, which will increase the permeability of water vapor, allowing the water to diffuse through the polymer more easily.
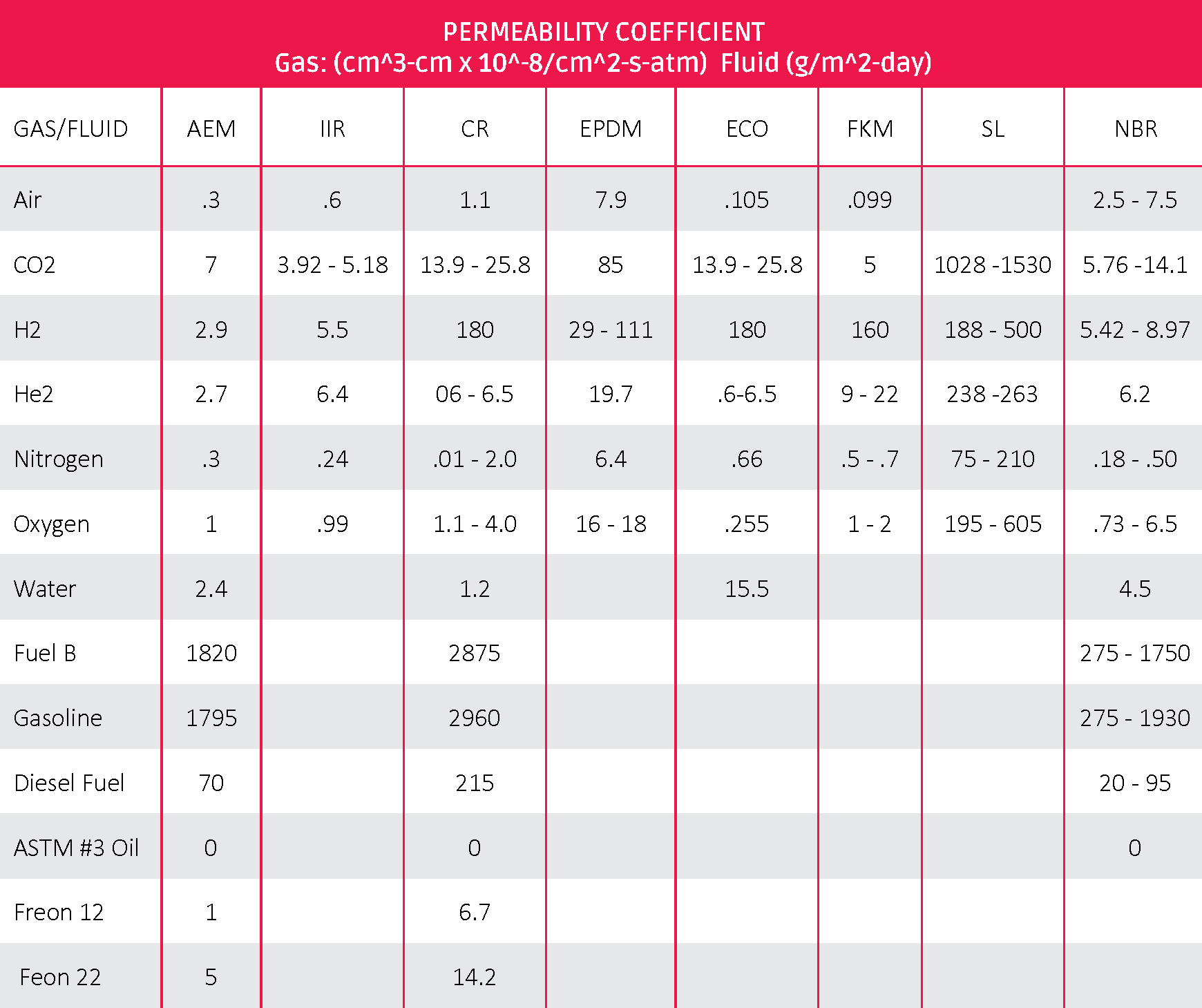
The polarity of the polymer. Different rubber base polymers have varying polarity. A helpful way to think of this is “likes dissolve likes.” This is why EPDM swells in oil or fuels. Both the EPDM polymer and the oils are nonpolar. NBR polymers have increasing levels of polarity, making them repeal oils and fuels and reduce swelling. Matching the polarity of rubber barriers and the polarity of the permeant is important.
Table 2: ASTM D1418 Rubber to Permeability Coefficient
The above numbers are based on standard con STD conditions. Higher temperatures will typically increase rate. For example, ranges of NBR are based on ACN content of the polymer. Higher ACN produces lower results for fuels and gases due to higher polarity.
These numbers should not be used for estimation purposes. Actual test data from the compound being used will provide more precise estimates.
Citations:
-
McKeen, L (2012). Permeability Properties of Plastic and Elastomers. Waltham, MA: Elsevier Inc.
-
Pruett, K (2005). Chemical Resistance Guide For Elastomers III. La Jolla, CA: Compass Publications